Dallas Researchers Test New Medication for Children with Migraine Headaches
Cedar Health Research enrolling pediatric migraine patients in clinical trials.
Every parent knows the pain of watching their child suffer from a headache. Our hearts break when their heads hurt. Some children can find relief after some simple at-home remedies like taking a nap and increasing hydration. Other children suffer from chronic and recurrent headaches known as migraines.
Dallas-based Cedar Health Research is enrolling local patients in two pediatric studies to expand the safe and effective treatment options for children with migraines. Cedar Health Research partners with physicians, like me, in the Dallas/Fort Worth Metroplex and across Texas to expand patient opportunities in clinical trial participation.
Migraine headache is a common childhood problem affecting up to 10% of children age 5-15 and up to 28% of teenagers. When simple measures do not ease the pain, some children require migraine relief medication such as acetaminophen or ibuprofen. Despite these treatments, many children still suffer excruciating pain and require prescription medication. The good news is approximately 25% of children will outgrow migraines by the age of 25. Until then, parents are searching for options to help.
Many drugs approved for adults have not been tested in children resulting in “off-label” use. The Food and Drug Administration initiated a pediatric program to allow well-controlled clinical trials to test the safety and efficacy of adult-approved medication in children.
Parents may find more effective migraine therapy options by enrolling a child in one of these clinical trials.
Headaches in Children
There are two common types of headaches that affect children. A pediatrician or pediatric neurologist will assess a child with recurrent headaches to determine the cause based on a series of diagnostic criteria.
1.Tension headaches. Tension-type headaches cause pain, pressure, or tightness on both sides of the head. The pain can be severe but usually does not interfere with daily activities.
2. Migraine headaches. Migraine headaches often start on one side and get worse over time. Children often have other accompanying symptoms like nausea, light sensitivity, dizziness, or visual changes. Other symptoms include mood changes, fatigue, anxiety, concentration problems, food cravings, neck stiffness, and hyperactivity. The pain can become progressively worse and keep the child from daily activities.
A doctor will help parents and children learn to recognize migraine signs and teach preventative and management steps.
The goal of migraine treatment is to find safe and effective pain relief to help the child return to normal functions as soon as possible. Headache calendars can help identify triggers that lead to migraines. Early interventions, such as taking a nap or resting in a dark room, may help prevent headaches. Lifestyle modifications like stress reduction, improving sleep habits, and dietary changes may help some children.
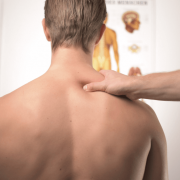
When these remedies are not effective, doctors will recommend over-the-counter medications such as acetaminophen and nonsteroidal anti-inflammatory drugs like ibuprofen and naproxen. For patients who continue to suffer from migraines, doctors may prescribe FDA-approved medications such as zolmitriptan and sumatriptan.
Image CC hih.gov
Rimegepant; A new option for pediatric migraines
Cedar Health Research, located in Dallas and Irving, is currently enrolling candidates in two clinical trials to evaluate the safety and tolerability of Rimegepant in children aged 6-18 with a history of migraines. Rimegepant, from Biohaven Pharmaceuticals, is an FDA-approved medication for adults for the acute treatment of Migraines.
Rimegepant helps adult patients find migraine relief by reducing calcitonin gene-related peptide (CGRP) induced symptoms. The drug’s mechanism of action blocks exaggerated pain signaling, inhibits blood vessel dilation, and reduces inflammation. Although Rimegepant is currently FDA-approved for adults, clinical trials are needed to determine children’s safety and efficacy.
Phase 1 trial
The phase one clinical trial evaluates the pharmacokinetics of Rimegepant to determine the appropriate weight-based dosing regimen. Participants receive one dose of medication, and then blood samples are performed at four intervals after dosing. This allows scientists to track the body’s absorption of the medicine.
All qualifying candidates will receive the medication and are compensated for their participation.
Candidates must meet eligibility criteria, including:
- Age 6-12 years old
- Weight greater than 15 kg (33 lbs)
- Participants must be able to read and comprehend written instructions
- Participants must have a medically documented 6-month history of migraines
- A qualifying score on the Sheehan Suicidality Tracking Scale (S-STS)
- Must be able to be tolerate blood draws
Phase 3 trial
The phase three trial is a multicenter, randomized, double-blind, placebo-controlled study to assess the efficacy and safety of Rimegepant for treating migraines in children age 6-18 years old. This study aims to test how well the study drug works in children compared to a placebo. Up to 1440 participants will undergo evaluation during a 19-week timeline to treat up to two migraines of moderate to severe intensity.
All qualifying candidates will be randomly assigned to receive the medication or placebo and are compensated for their participation.
Candidates must meet eligibility criteria including:
- Age 12 and 18 years old.
- Participants must experience at least one migraine a month.
- Participants must have a history of migraines for at least 6 months.
Learn more about the FDA Clinical trial process here in this video from the Food and Drug Administration.
Article originally published by Newsbreak.
Blog Author: Dr. Jeff Livingston
Main Blog Photo By: OlgaVolodina Istock by Getty
Disclosure: The author serves as a principal investigator and sub-investigator in Women’s health studies through Cedar Health Research.