FDA Approves Covid-19 Pfizer Vaccine for Kids Age 12–15
Great news for teens who are ready to get back to normal.
Just in time for summer, the US Food and Drug Administration (FDA) announced game-changing news. The FDA expanded the emergency use authorization for the Pfizer messenger RNA Covid-19 vaccine for kids age 12–15 years old.
The Pfizer-BioNTech COVID-19 Vaccine met the FDA’s safety and efficacy criteria to expand the emergency use authorization. Until now, the Pfizer vaccine was approved for use in people 16 years and older.
Pfizer previously announced in a press release highly encouraging results from their Phase 3 clinical trial. The Pfizer-BioNTech COVID-19 vaccine was 100% effective and generated a high antibody response in children aged 12–15.
The Phase 3 study included 2,260 US participants. The research detected only 18 cases of Covid-19, and all were in the placebo group. None were in the study participants who received the vaccine. The Pfizer vaccine was 100% effective in preventing Covid-19 in this age group.
The side effect profile in children was similar to those in adults. Injection site pain, fatigue, headache, chills, muscle pain, fever, and joint pain were the most common side effects.
Until recently, infection rates in children have been low. Children are often asymptomatic carriers but can pass the infection on to parents, teachers, and grandparents. The rise of variants such as B.1.1.7 is changing the way we view Covid-19 in children.
A March outbreak linked to youth sports in Minneapolis was a public health wake-up call. Health policymakers noted how fast Covid-19 infections in children can spread to the general public.
The FDA press release reports the CDC has confirmed 1.5 million Covid-19 cases in children age 11–17. The American Academy of Pediatrics data shows over 3.85 million children have tested positive for COVID-19 since the onset of the pandemic.
Fortunately, most children infected with SARS-CoV-2 do well. Children account for only 0.00%-0.21% of all Covid-19 deaths.
Vaccinating children helps prevent the further spread of Covid-19 and helps the US move closer to herd immunity.
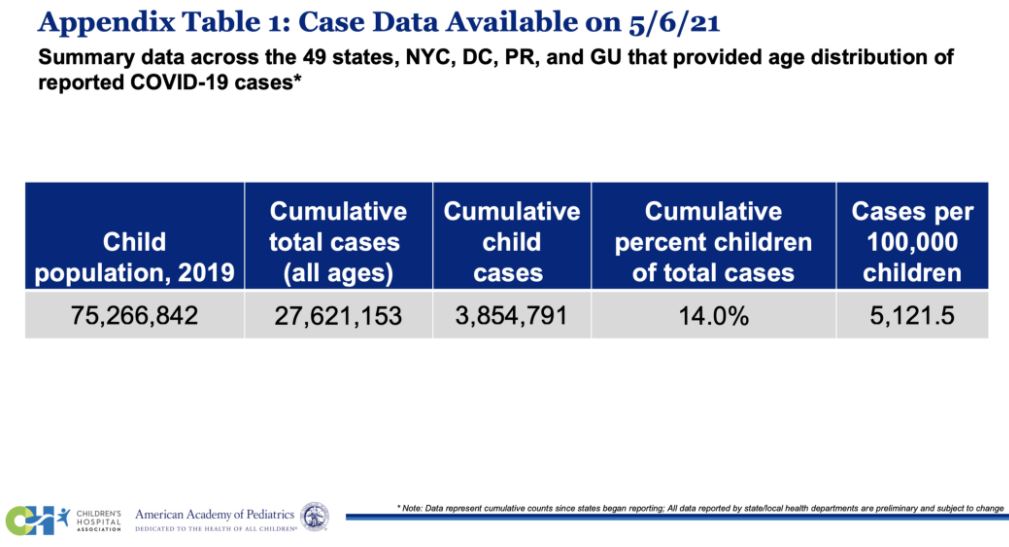
Chart: CC American Academy of Pediatrics
Like the Moderna and Johnson and Johnson vaccines, the Pfizer vaccine does not contain a live virus. One cannot catch Covid-19 from these vaccines. The mRNA vaccines do not enter our cells’ nucleus and do not alter vaccine recipients’ DNA.
These vaccines do not use an adjuvant to enhance vaccine efficacy. An adjuvant is an agent used to provoke a more robust immune response. These vaccines do not make use of adjuvants.
Immunity does not come immediately after vaccination. It takes time for your body to build up protection. The Pfizer mRNA vaccine requires two doses. The first shot primes the immune system to produce protective antibodies. The second dose kicks it into high gear.
Here is what we know about post-vaccine immunity with the two Covid-19 vaccines currently available in the U.S.: In phase 3 clinical trials, the Pfizer vaccine showed a 95% efficacy seven days after the second dose. The Moderna vaccine offers 94% immunity at least 14 days after dose number two.
Two weeks after completing the vaccination course, recipients can breathe a sigh of relief. Their risk of severe disease from Covid-19 is very low. But we must remember that the risk is not zero.
Want to see what getting a Covid-19 vaccine looks like? Here is what happened when I got mine.
Article originally published on Medika Life.
Blog Author: Dr. Jeff Livingston
Main Blog Photo By: DisobeyArt Istock/ Getty Images